Info
Quick User Guide for LYNXERA® Microtome Blade Models
Manufacturer:
LUTZ GmbH & Co. KG, Piepersberg 20, 42653 Solingen, Germany
We are a German blade manufacturer with over 100 years of expertise in crafting blades for the most demanding cutting needs.
Intended Use:
LUTZ‘s microtome blades from Solingen serve as cutting tools in microtomes for creating thin tissue samples in histological examinations. Therefor, insert the blade into a microtome and cut prepared samples. They are considered cutting tool accessories for diagnostic equipment and comply with EU regulations for in-vitro diagnostics.
Usage Instructions:
Blades are cutting tools with sharp edges, as commonly known. Please handle the blades with care, just like any other cutting tool. The blade dispenser provides individual blades by operating the slider.
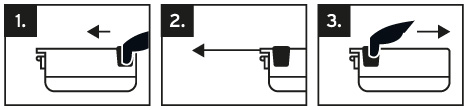
Refer to the pictogram sequence for guidance
Gently insert the blade into the microtome, avoiding contact between the blade edge and microtome components. When using the blades, we recommend using hand and eye protection, although it‘s not mandatory. Exercise caution, similar to handling other cutting tools. Please ensure that the blade dispensers are stored in a dry environment both before and during use.
Disposal Instructions:
The blades remain sharp even after use. Please dispose the blades appropriately in the disposal container located at the bottom of the dispenser, marked “Used Blades”, or in a suitable waste container within your laboratory equipment.
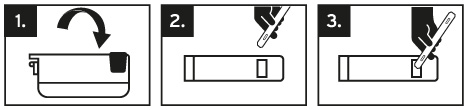
Die Piktogrammfolge zeigt Ihnen den Vorgang der Klingenentsorgung im Behälter „Used Blades“
Technical Specifications:
Material: High-alloy martensitic stainless steel with a high chromium content.
Permissible temperature range during transport: -10°C to +50°C
Permissible operating temperature of the blade dispenser: +10°C to +40°C
Operating temperature of the blades in the microtome: -40°C to +40°C
Warranty Commitment:
LYNXERA® brand blades are manufactured in Germany and adhere to the highest standards of accuracy and consistent quality throughout the production process. This commitment ensures process capability for your cutting applications in the laboratory. We take our responsibility seriously, providing you with a cutting tool of the utmost precision and sharpness. If you are not satisfied with our blades, please contact us, mentioning the REF number on the packaging.
We are here to assist you. Our goal is your success!

Manufacturer

Reference number

Production date

In-vitro diagnostics

Declaration of conformity
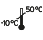
Temperature limits during transport
FOR-0870 Rev.001/29.09.23
